how to find molecular formula from percent composition
Pct means one part in a 100 or part in a whole. Therefore, percent composition of a chemical compound is the percent by mass of each element in the total mass of a compound. Another fashion of saying the same affair is how much in percentage each element contributes to the total mass of a compound. The higher the percentage composition, the higher the mass of the element present in the compound. Mathematically, we can limited percent composition as:

At present, let's use the in a higher place formula to calculate the percentage limerick of each element in water— H2O. From the chemical formula of h2o, we know that 1 mole of water contains 2 mol of hydrogen, H and i mol of oxygen, O. That is, we are assuming that:
ane mol of HtwoO contains 2 mol of H and ane mol of O. But we do know that the molar mass of hydrogen is about ane.01 g/mol and the molar mass of oxygen is most 16.00 g/mol. Therefore, nosotros can calculate the mass of 1 mol of water, H2O as:
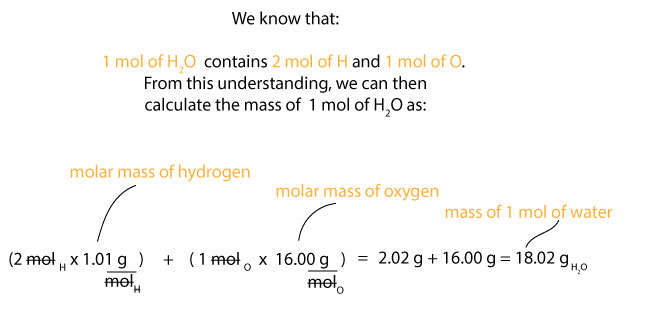
At present that we know the molar mass of h2o (whole), we can and so calculate the percentage composition of hydrogen and oxygen in it as follows. For hydrogen, we know that its tooth mass is ane.01 k/mol, merely since we take 2 mol of it in water, and so it follows that we must multiply the 2 mol of H past its tooth mass. If we do, we will get ii.01 g, which is as a upshot of doing this: 2 mol ten 1.01 g/mol = ii.02 grand. Nosotros tin can and so use this value, the molar mass of h2o, and the percentage limerick formula depicted in equation 1 above to summate the percentage limerick of hydrogen in water. If yous are following along, y'all will observe that we tin set this adding as:

Similarly, for oxygen, nosotros know that its molar mass is 16.00 g/mol, but since nosotros take i mol of it in water, then it follows that we must multiply the i mol of O past its tooth mass. If we practice, nosotros will become 16.00 g, which is equally a effect of doing: one mol x 16.00 g/mol = 16.00 g. We can then use this value, the molar mass of water, and the percentage limerick formula depicted in equation 1 to a higher place to calculate the percentage composition of oxygen in h2o. If yous are post-obit along, you lot will notice that nosotros can fix this calculation as:

From the calculation, you can run across that the pct composition of hydrogen and oxygen in water are 11.21% and 88.79% respectively. If you add these values together, you will notice they sum up to 100%. This ways regardless of whether water is from mars or from earth, once it is pure h2o, its composition is fixed and will always consist of xi.21% H and 88.79% O.
Notice!
Since we know that you will become 100% when you add the percentage composition of all the elements in a chemical formula, so it follows that we tin subtract the percentage composition of H from 100% to become that of oxygen. If you do, you will get:
- 100% -eleven.21% = 88.79%.
Once you know the percentage limerick of all the elements in a chemic formula, except 1. You can always use this shortcut to detect its percentage limerick.
Source: https://masterconceptsinchemistry.com/index.php/2019/03/02/percent-composition-what-is-it-and-how-to-calculate-it-from-a-chemical-formula/
Posted by: fieldsforomed.blogspot.com

0 Response to "how to find molecular formula from percent composition"
Post a Comment